Introduction:
Newborn screening (NBS) for sickle cell disease (SCD) has significantly improved childhood survival. However, gaps remain in follow-up after NBS as demonstrated by poor implementation of penicillin prophylaxis and stroke risk (TCD) screening. It is unknown how time to initial engagement with SCD specialist may impact long-term patient engagement. Currently, there are no standardized follow-up requirements for NBS across the United States; each state can decide how they inform families of diagnoses and initiate follow-up. The average time to the initial follow-up with a SCD specialist is not currently evaluated as a measure of quality assurance.
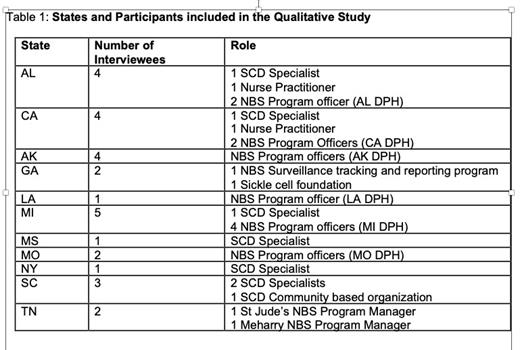
Semi-structured interviews were conducted with 19 participants across 11 states to explore NBS processes. Participants included health care providers (n = 7), and stakeholders (NBS health department employees and community based SCD organization representatives (CBO); (n = 12). Data were analyzed using directed content analysis, informed by the SCD care continuum model, which outlines how newborns with SCD should proceed from diagnosis to initial and ongoing care engagement with a SCD provider. We examined how families were informed of abnormal results, how newly-diagnosed children were referred to SCD specialists, and when cases were closed.
Results
Representatives from 11 states including AL, AK, CA, GA, LA, MI, MS, MO, NY, SC, TN were included in this study (Table 1).
Initial Notification: In all 11 states, the physician of record (on the NBS bloodspot card) is notified of abnormal results and expected to communicate results to families. This physician of record is not necessarily the child's actual pediatrician. In 2 states, a letter is sent to families at the same time as physicians are notified, but the letter does not convey screening results. In 1 state, the CBO NBS coordinator is responsible for communicating results to the family.
Referral to a SCD specialist: There was substantial variability in referral practices. In most states, infants with positive SCD screens were referred to a SCD specialist by the pediatrician. In some states, the public health department refers infants with positive screens directly to the SCD center. In 2 states, the decision to refer an infant to a SCD center is left to the family, NBS coordinator or CBO.
Timing of first visit: Most states intend infants with positive SCD screens to follow-up with the SCD clinic in < 2 months; however, some states do not require the initial visit until 6 months. Notably, most states do not track whether children with a positive SCD screen ever see a SCD specialist, Instead, cases are closed once the physician of record is informed.
Facilitators and Barriers: The most common facilitators for communicating NBS results were having the correct physician of record on the blood spot card, the physician's knowledge of SCD, or the use of state-based immunization databases to track children with missing demographic information. Key facilitators for linkage to care included partnerships with SCD CBOs and/or having designated NBS follow up coordinators that offer ongoing family education and appointment reminders. Other facilitators included families with extended support and those who already had a child with SCD.
The most common barriers to communicating NBS results were difficulty locating or reaching families, families moving out of state, insufficient contact information, and/or having the wrong physician of record. The main barriers to linkage to care included lack of insurance, lack of transportation, families who have difficulty accepting the SCD diagnosis, immigrant or refugee families with language barriers and other conflicting social/life demands.
Conclusion
The results of our study demonstrate wide variability in NBS follow-up for SCD with limited quality assurance to ensure newborns see a SCD specialist. These findings indicate a large multi-level intervention trial is needed to identify best practices for systematic improvement in NBS follow up process and ensure all infants are seen by a SCD specialist.
Disclosures
Kanter:Bausch: Consultancy; Glycomimetics: Membership on an entity's Board of Directors or advisory committees; Fulcurm: Consultancy; ECOR-1: Consultancy; Guidepoint Global: Honoraria; Vertex: Consultancy; Chiesi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; BEAM: Consultancy, Research Funding; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; HRSA: Research Funding; CDC: Research Funding; NHLBI: Research Funding; Austin Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Watkins, Lourie, Roll & Chance: Consultancy; National Alliance of Sickle Cell Centers: Other: President.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal